

When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.įinding molar mass starts with units of grams per mole (g/mol).
LEAD ATOMIC MASS NUMBER HOW TO
This site explains how to find molar mass. The reason is that the molar mass of the substance affects the conversion. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. 8 Molar Mass and Numbers of Molecules The equivalence point of the titration is the point when the moles of H+ are equal to. To complete this calculation, you have to know what substance you are trying to convert. Element Lead (Pb), Group 14, Atomic Number 82, p-block, Mass 207.2. These relative weights computed from the chemical equation are sometimes called equation weights.Ī common request on this site is to convert grams to moles. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.įormula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. Also note that 3 of the 4 stable isotopes also have even numbers of. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. Lead has an even number of protons so having four stable isotopes is not unexpected. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. The atomic number of an element is equal to the number of protons and electrons in that element. Since the iodine is added as a 1 anion, the number of electrons is 54. Lead is the 82nd element of the periodic table so its atomic number is 82. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 53 74). This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. The atomic number of iodine (53) tells us that a neutral iodine atom contains 53 protons in its nucleus and 53 electrons outside its nucleus. Lead (Pb) is a metal which has been used by humans for centuries dating back to 7000 BC. The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. Lead is an element of the periodic table with an atomic number of 82. Other atoms don’t generally have round-number atomic masses for reasons that are a little beyond the scope of this article. By definition, an atom of carbon with six neutrons, carbon-12, has an atomic mass of 12 amu. The known elements are arranged in order of increasing Z in the periodic table (Figure 1.6.1). The atomic mass of a single atom is simply its total mass and is typically expressed in atomic mass units or amu. The atomic number is therefore different for each element. Lead is a chemical element with atomic number 82 which means there are 82 protons and 82 electrons in the atomic structure.

LEAD ATOMIC MASS NUMBER FREE
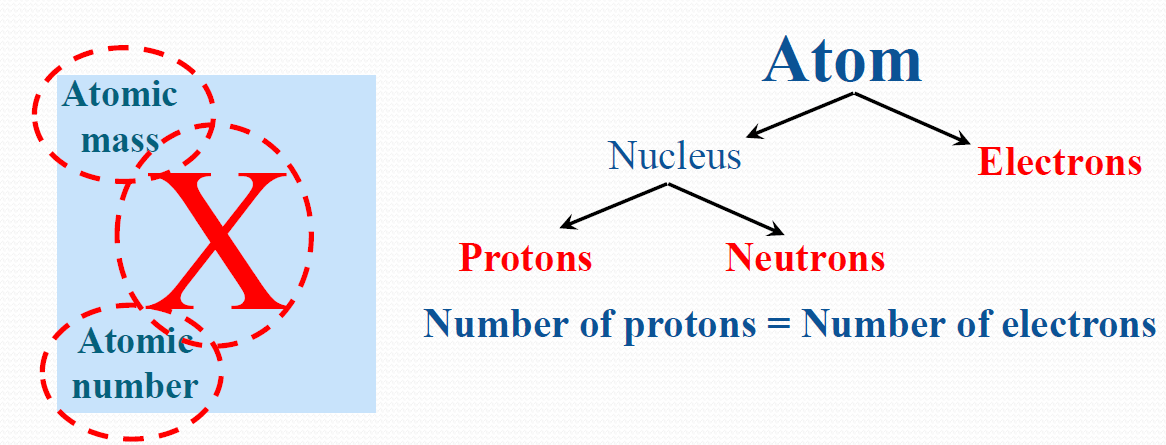
Free Vector Bookmark icon Collection Heart icon Favorite Share icon Share. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. The identity of an element is defined by its atomic number (Z), the number of protons in the nucleus of an atom of the element. Chemical symbol with atomic number and atomic mass.


 0 kommentar(er)
0 kommentar(er)
